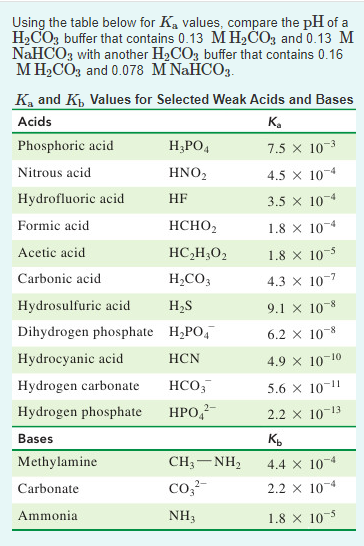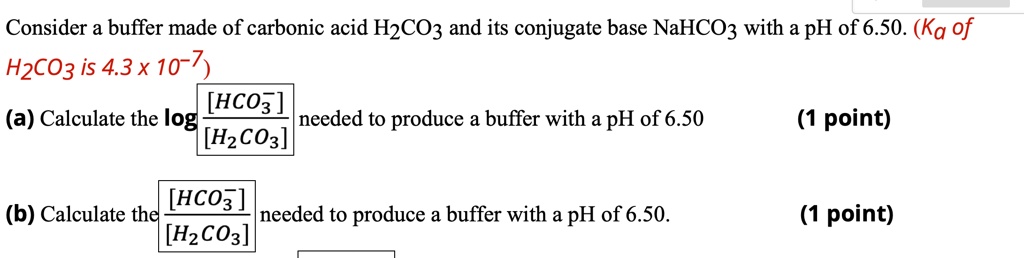
SOLVED: Consider a buffer made of carbonic acid HzCO3 and its conjugate base NaHCO3 with a pH of 6.50. (Ka of H2CO3 is 4.3x10-7) [HCO3 (a) Calculate the log needed to produce
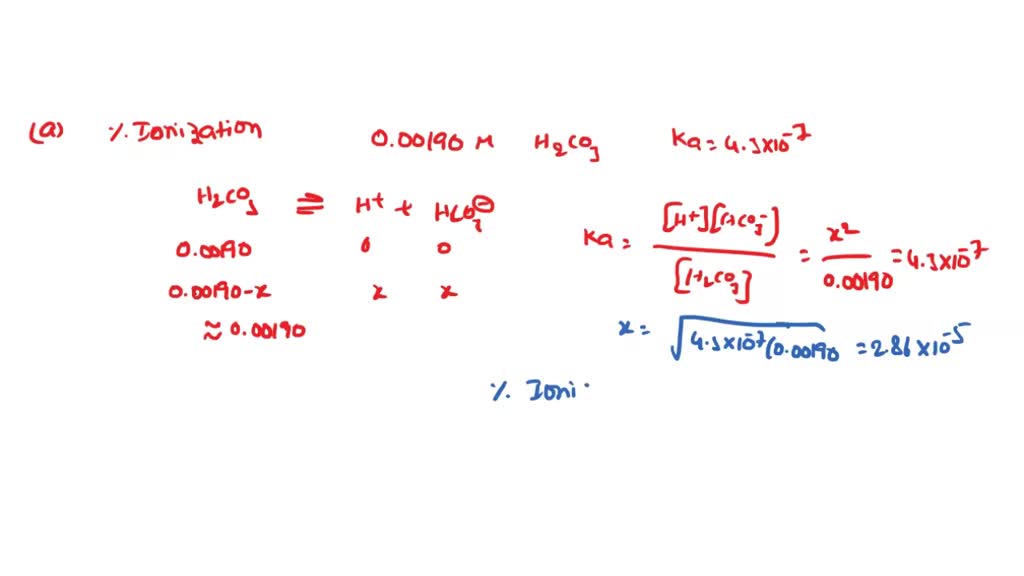
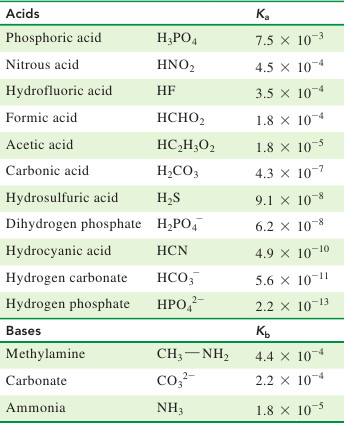
SOLVED: Calculate the percent ionization of carbonic acid (H2CO3) in solutions of each of the following concentrations (Ka = 4.3e-07.) (a) 0.281 M % (b) 0.366 M % (c) 0.641 M %

Sejumlah H2CO3 (Ka = 4,3 x 1O-7) dicampurkan dengan larutan Ca(OH)2 membentuk larutan penyangga. Setelah - Brainly.co.id

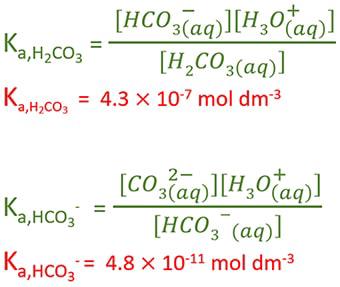
OneClass: Complete the Ka1 expression for H2CO3 in an aqueous solution. Do not include states in the ...

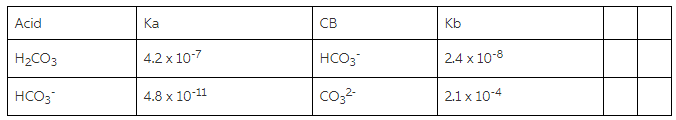
For carbonic acid the Ka1 = 4.30 × 10^-7 and the Ka2 = 5.62 × 10^-11. Calculate the pH of a 0.15 M solution of Na2CO3 :

SOLVED: The blood buffer system is composed of H2CO3 (carbonic acid, Ka = 7.9 x 10^-7 ) and its conjugate base, HCO3- (bicarbonate). In a healthy adult, the pH of blood is

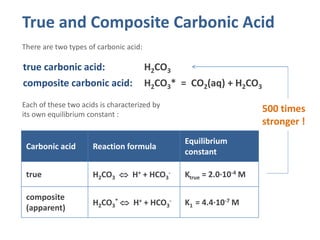
The Ka of carbonic acid is 4.3 x 10-7. H2CO3 = H+ + HCO3 This means that H2co3 is a____. A.good - Brainly.com
Why is carbonic acid a weak acid even though it gets completely dissociated into H+ and CO3- ions? - Quora
Find the concentration of H+, HCO^-3 and CO^2-3, in a 0.01M solution of carbonic acid if the - Sarthaks eConnect | Largest Online Education Community
![Carbonic acid (H(2)CO(3)), a diprotic acid has K(a1)=4.0xx10^(-7) and K(a2)=5.0xx10^(-11). What is the [CO(3)^(2-)] of a 0.025 M solution of carbonic acid? Carbonic acid (H(2)CO(3)), a diprotic acid has K(a1)=4.0xx10^(-7) and K(a2)=5.0xx10^(-11). What is the [CO(3)^(2-)] of a 0.025 M solution of carbonic acid?](https://d10lpgp6xz60nq.cloudfront.net/ss/web/477725.jpg)
Carbonic acid (H(2)CO(3)), a diprotic acid has K(a1)=4.0xx10^(-7) and K(a2)=5.0xx10^(-11). What is the [CO(3)^(2-)] of a 0.025 M solution of carbonic acid?