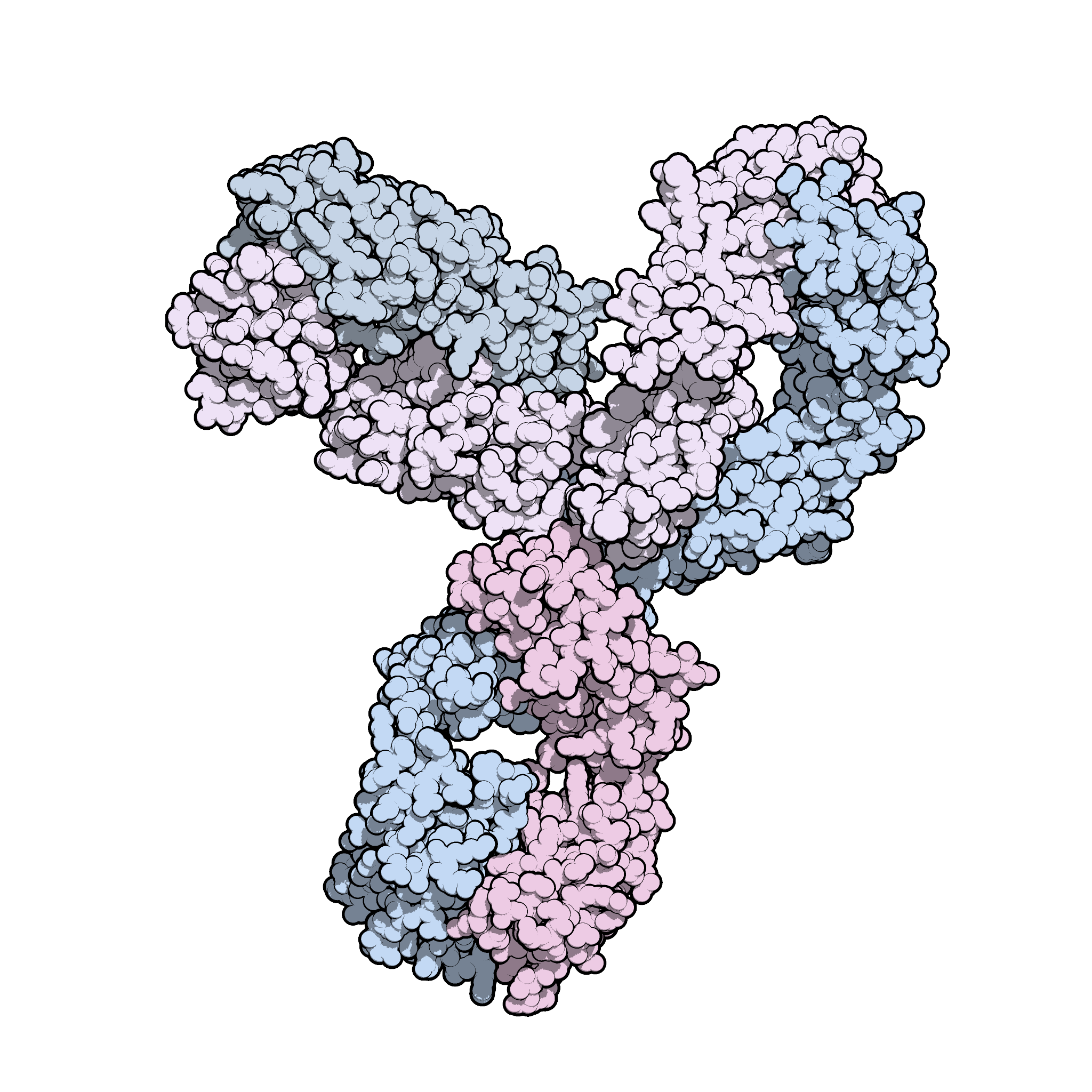
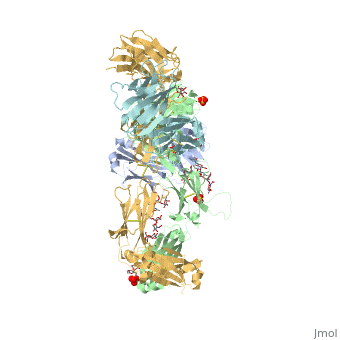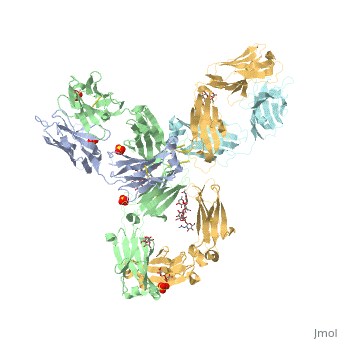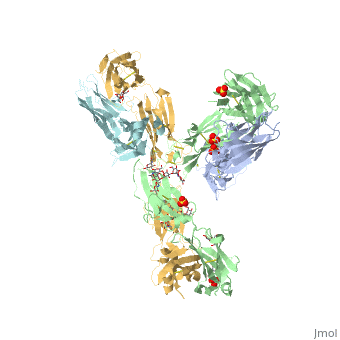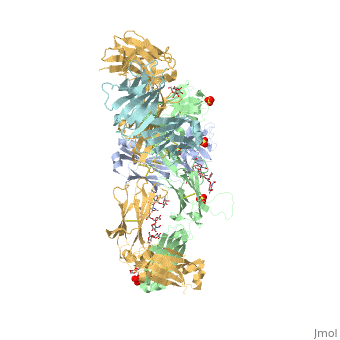Updated Analysis of KEYNOTE-024: Pembrolizumab Versus Platinum-Based Chemotherapy for Advanced Non–Small-Cell Lung Cancer With PD-L1 Tumor Proportion Score of 50% or Greater | Journal of Clinical Oncology
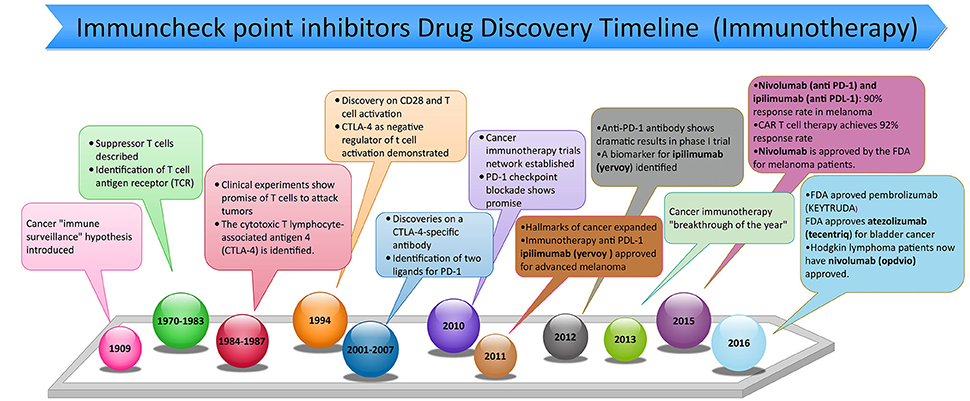
Frontiers | PD-1 and PD-L1 Checkpoint Signaling Inhibition for Cancer Immunotherapy: Mechanism, Combinations, and Clinical Outcome

Zinc complex of 3,5-di-tert-butyl salicylate inhibits viability, migration, and invasion in triple-negative breast cancer cells | Scientific Reports

FDA Approves Merck's KEYTRUDA® (Pembrolizumab) for First-Line Treatment of Patients with Unresectable or Metastatic MSI-H or dMMR Colorectal Cancer, First Single-Agent, Anti-PD-1 Therapy Approved for the First-Line Treatment of These Patients -
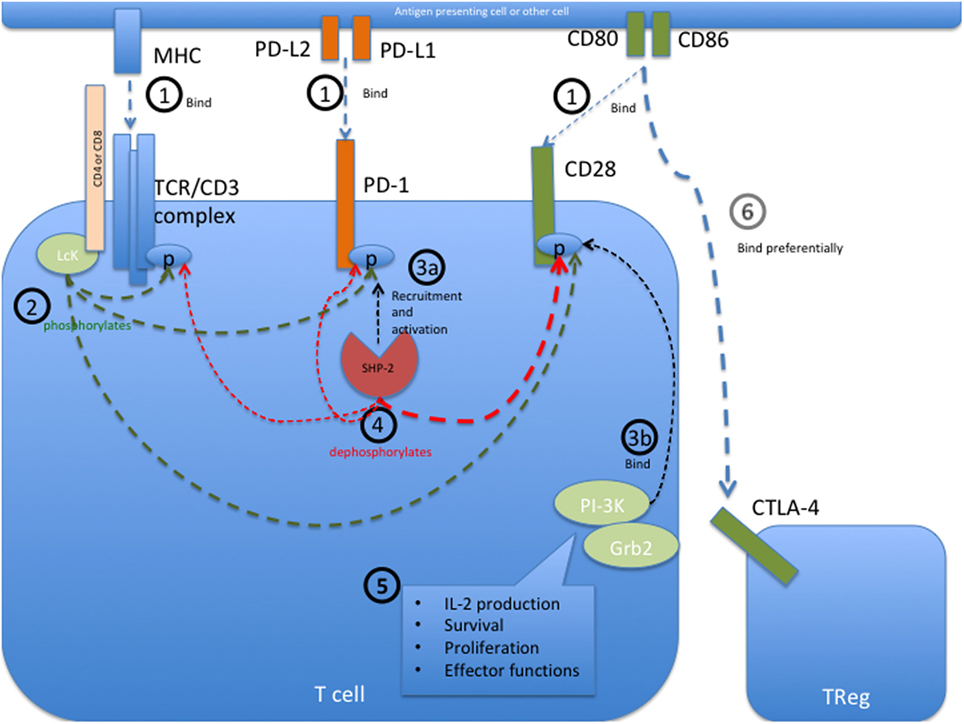
Frontiers | Anti-PD-1 and Anti-CTLA-4 Therapies in Cancer: Mechanisms of Action, Efficacy, and Limitations
Merck receives EC approval for Keytruda to treat relapsed Hodgkin Lymphoma - Pharmaceutical Technology
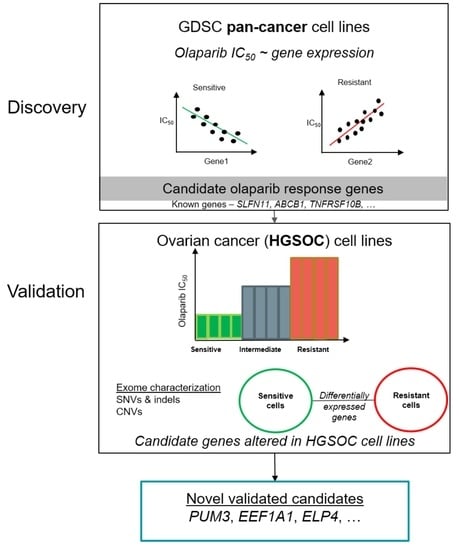
Cancers | Free Full-Text | Candidate Markers of Olaparib Response from Genomic Data Analyses of Human Cancer Cell Lines